
Alright, let's go ahead and calculate the wavelength of light that's emitted when the electron falls from the 3rd energy level to the 2nd. And, so, that's how we calculated the Balmer Rydberg equation in the previous video. Alright, so, if an electron is falling from n is equal to 3 to n is equal to 2- Let me go ahead and draw an electron here- So, an electron is falling from n is equal to 3 energy level down to n is equal to 2, and the difference in those two energy levels, are the difference in energy is equal to the energy of the photon. And, now, let's see if we can calculate the wavelength of light that's emitted. So, let's look at a visual representation of this. Alright, so it's going to emit light when it undergoes that transition. So, we have an electron that's falling from n is equal to 3 down to a lower energy level, n is equal to 2. For example, let's say we were considering an excited electron that's falling from a higher energy level. And, you can see that 1 over Lambda, Lambda is the wavelength of light that's emitted, is equal to R, which is the Rydberg constant, times 1 over i squared, where i is talking about the lower energy level, minus 1 over j squared, where j is referring to the higher energy level. So, I call this equation the Balmer Rydberg Equation.

And, we can do that by using the equation we derived in the previous video. And, since line spectrum are unique, this is pretty important to explain where those wavelengths come from. And so, this emission spectrum is unique to hydrogen and so this is one way to identify elements and so this is a pretty important thing. You'll also see a blue-green line, and so this has a wavelength of 486 nanometers a blue line, 434 nanometers and a violet line at 410 nanometers. So, you see one red line, and it turns out that that red line has a wavelength- that red light has a wavelength of 656 nanometers. So, this is the line spectrum for hydrogen. So, since you see lines, we can call this a line spectrum. If you use something like a prism or diffraction grading to separate out the light, for hydrogen you don't get a continuos spectrum. And so, we talked about this in the last video. When those electrons fall down to a lower energy level, they emit light. So, if you passed a current through a tube containing hydrogen gas, the electrons and the hydrogen atoms are going to absorb energy and jump up to a higher energy level. If you did a similar thing with hydrogen, you don't see a continuos spectrum. It's continuous because you see all of these colors right next to each other, so they kind of blend together, so that's a continuos spectrum.
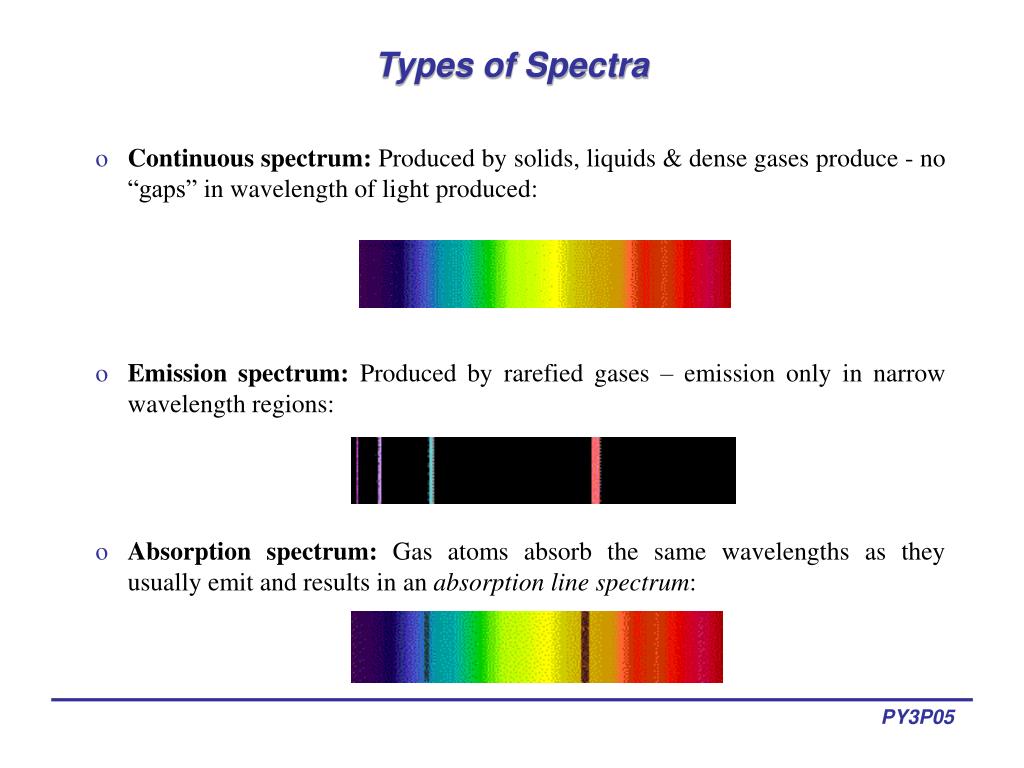
I'm going to call this a continuous spectrum. And so, if you do this experiment you might see something like this rectangle up here, so all of these different colors of the rainbow. I'm sure that most of you know the famous story of Isaac Newton, where he took a narrow beam of light and he put that narrow beam of light through a prism, and the prism separated the white light into all the different colors of the rainbow.


 0 kommentar(er)
0 kommentar(er)
